Spread the word !
Other posts
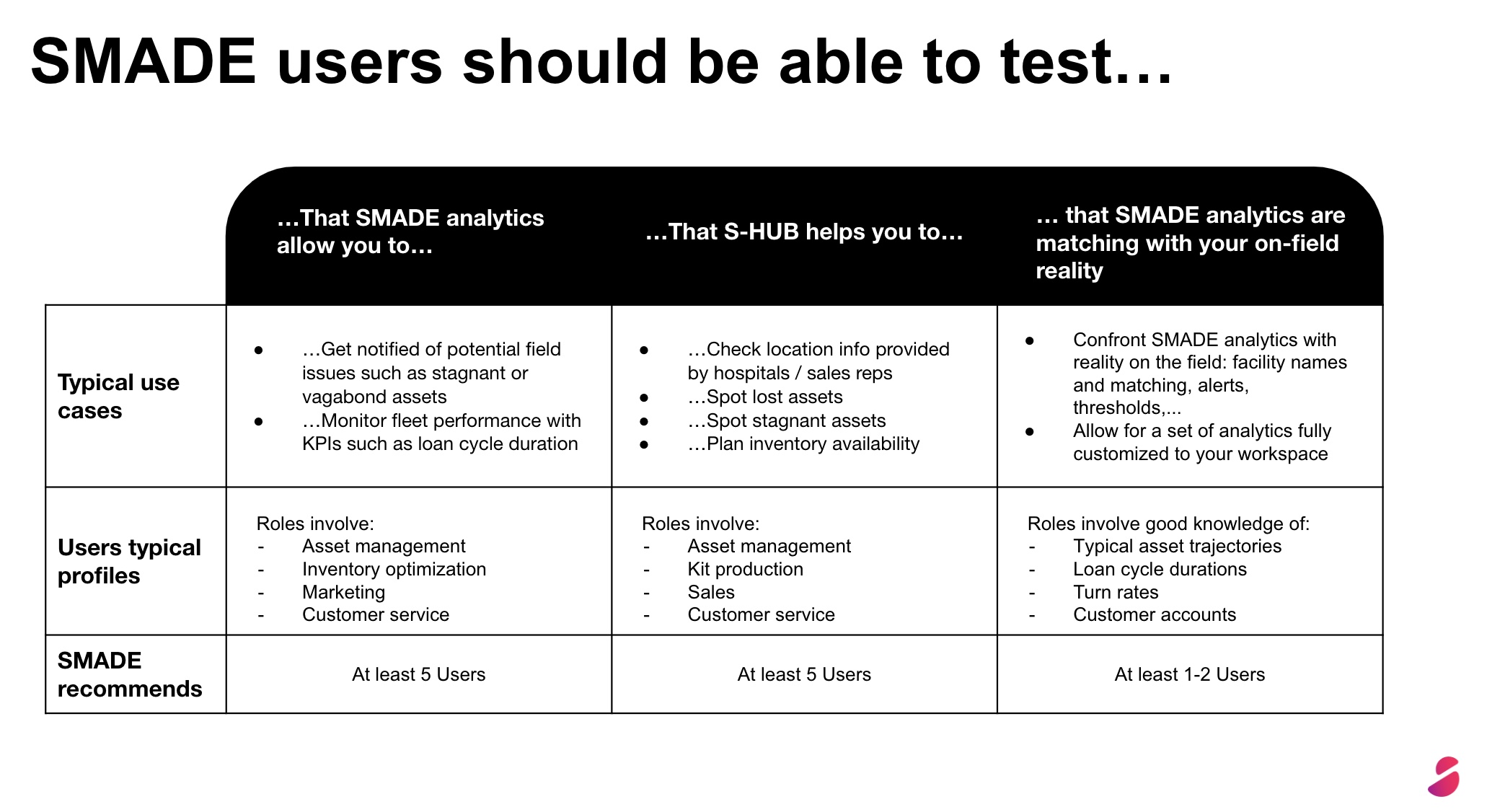
How can data drive continuous improvement?
Orthopedic medical devices account for 7.5% of the global medical devices market and is expected to grow owing to a globally increasing patient pool…
How to predict the future of the healthcare industry without relying on a crystal ball?
SMADE, a solution for cost optimizationWith the healthcare digital transformation comes the proliferation of connected devices and software…
If you love to write about smart healthcare, fill this form to become a writer for ‘In Your hands’.