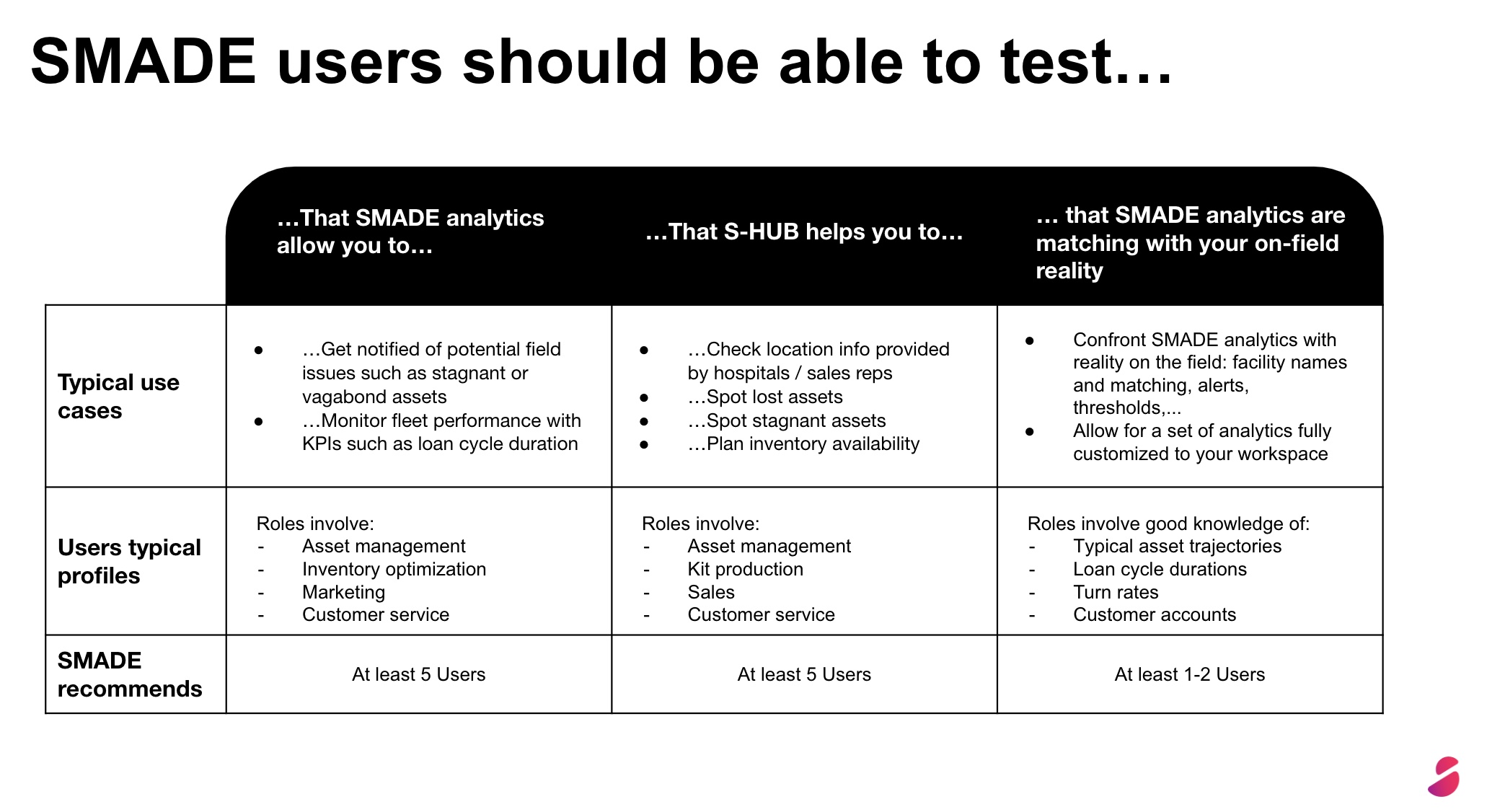
The smart medical instrumentation industry is evolving in a changing regulatory environment. In Your Hands provides updated information on main regulations that impact the sector.
May 20, 2021
Unique Device Identification (UDI) requirements : turning regulatory constraints into business opportunities
According to the new EU medical devices regulation, all medical devices must carry a “unique device identifier” – a unique alphanumeric code,…
If you love to write about smart healthcare, fill this form to become a writer for ‘In Your hands’.